You’ve probably already heard of Polycystic Ovary Syndrome (or PCOS). It has a surprisingly high level of incidence, affecting close to 25% of Indian women between the ages of 18 and 44.
PCOS is classified as a ‘syndrome’ because it’s characterised by a set of associated symptoms. Researchers have not identified the starting point of it all, or a clearly defined reason behind it.
This means that in order to understand the condition, we need to take a holistic view of the way the menstrual cycle normally functions, where it goes wrong and its associated factors.
Let’s begin.
The normal functioning of the ovaries
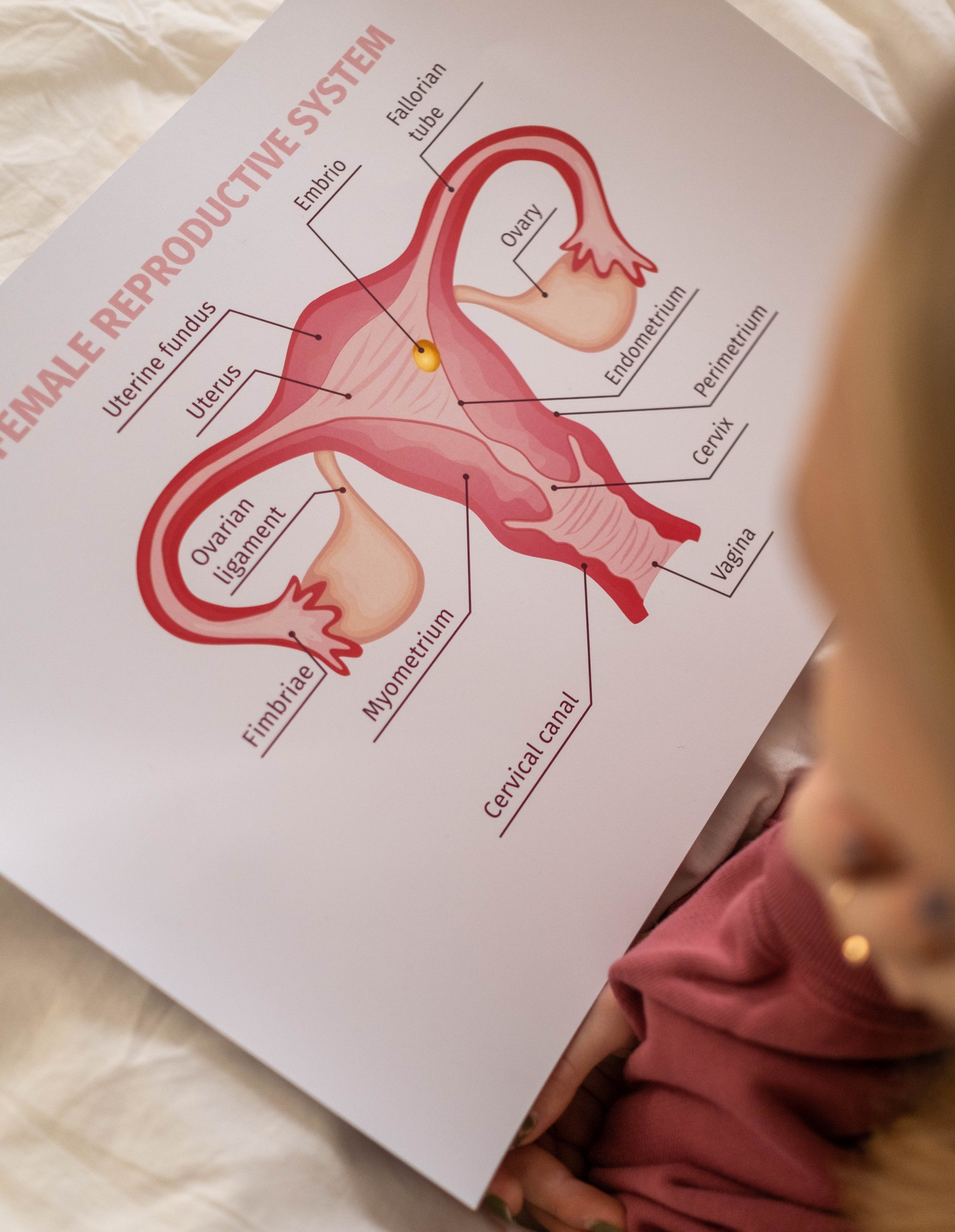
To understand PCOS, it helps to delve deeper into how the ovaries function.
The ovaries are a pair of female reproductive organs in which the ova, or eggs, are formed. The egg cells lie in small pockets called follicles. The cells lining the follicle multiply to form a layer (known as the zona granulosa) that contains granulosa cells.
The egg matures and gets released from the follicles during ovulation. For human females, the ovaries contain a number of these immature follicles right from birth.
After puberty, the follicles mature into primary and, later, secondary follicles. The cells surrounding the secondary follicle arrange themselves concentrically and form an enclosing sheath called theca cells- these play a vital role in the subsequent stages of ovulation. They produce hormones such as androgens (hormones that regulate male characteristics) and, from these androgens, the primary female sex hormones called oestrogens that influence fertility.
The 5 reproductive hormones & their effects on menstruation
There are five main hormones that control the reproductive cycle. Three of them are produced in the brain (GnRH, FH and LH), while the other two (oestrogen and progesterone) are made in the ovaries.
The first, GnRH (Gonadotrophin-releasing hormone), is produced and secreted by nerve cells in the hypothalamus and is carried by blood vessels to the pituitary gland, which is located at the base of the brain and regulates the body’s hormones. Here, GnRH stimulates the pituitary to secrete FSH (follicle-stimulating hormone) and LH (luteinising hormone), both of which regulate ovulation.
FSH, as indicated by its name, stimulates the follicles, specifically the primary ovarian follicles, to develop into secondary follicles. LH helps the theca cells develop around the secondary follicles. Once matured, the theca cells begin to produce androgen hormones.
The androgens hormones are transported to the granulosa cells. These cells are stimulated by FSH, to convert androgens (with the help of an enzyme called aromatase) to the fourth female reproductive hormone, oestrogen.
When produced, oestrogen lowers FSH levels, so that the follicles stop growing and only the best-developed follicle will eventually go on to release an egg. Meanwhile, when oestrogen production reaches a certain level, it sends a message to the pituitary to secrete more LH into the ovaries, which creates a surge of LH that leads to the ovulation of the egg. The egg is now released into the fallopian tube.
Meanwhile, the remaining follicles in the ovaries form a structure called the corpus luteum, designed to produce progesterone, the fifth reproductive hormone. Its role is to prepare the endometrium (the lining of the uterus) for the possibility of the egg’s fertilisation.
If the egg isn’t fertilised, the corpus luteum breaks down, which stops the production of progesterone and leads to menstruation: the disintegration of the endometrium, which flows out through the vagina.
What goes wrong during PCOS
Although androgens are largely understood to be ‘male’ or masculising hormones, it’s important to note that they play a vital role in women as well. Androgens such as androstenedione and testosterone are needed to make the female hormones oestrogen and progesterone, help strong bones and lean muscles develop, and also promote a woman’s libido.
Polycystic ovaries develop when excessive amounts of androgens are produced by the ovaries and/or adrenal glands, or when there is an issue with the functioning of the hormone insulin.
Let’s take a closer look at each.
Excess androgen production
In the ovaries –
As mentioned earlier, the secretion of the hormones LH and FSH is controlled by the hormone GnRH. The hypothalamus secretes this in a pulsating manner; if the pulses are slow, the pituitary secretes more FSH than LH, and when they are fast, there’s more LH produced than FSH.
We aren’t sure why, but in PCOS, GnRH pulses are faster than usual, leading to excessive LH levels (as we know, LH normally stimulates the theca cells of the ovarian follicle to produce androgens, which with the help of FSH is converted to oestrogens in the granulosa).
Excess LH levels lead to the excessive secretion of androgen in the theca cells. When in high amounts, androgen prevents the enzyme aromatase from converting it into oestrogen in the granulosa cells. This impairs the development of ovarian follicles and slows down the production of oestrogen.
Without the development of the follicles, the egg is unable to develop (called anovulation). This leads to menstrual disorders and possibly infertility.
From the adrenal glands –
Apart from in the ovaries, excess androgens can also be produced due to issues with the adrenal glands. When we are stressed, our pituitary signals our brain to secrete the hormone ACTH (Adrenocorticotrophic hormone).
This hormone stimulates the adrenal glands to produce adrenaline and cortisol (stress hormones), as well as adrenal androgens (such as DHEA, DHEA-S and androstenedione). These adrenal androgens can be converted to testosterone, further contributing to the androgen excess.
Insulin dysfunction
Insulin’s primary role in the body is to help cells take up glucose from the bloodstream, for energy production and storage. It does this by binding to insulin receptors, found on the surfaces of most of our cells. When insulin binds to this receptor, the receptor activates a protein called IRS-1, which begins the cascade of signals that allow glucose to enter the cell.
Insulin resistance is caused when a bad diet, stress and chronic inflammation modify IRS-1 (by changing the structure of a specific amino acid in its structure, in a process known as serine phosphorylation). When this happens, IRS-1 cannot efficiently signal the cell to take up glucose.
At least 50% of women with PCOS appear to have insulin resistance.
A further complication of insulin dysfunction is that excess insulin inhibits the production of SHGB (sex hormone binding globulin), a protein that’s meant to bind to testosterone and making it inactive. This further contributes to the excess levels of androgens in an individual.
Issues that contribute to insulin resistance (such as obesity, a high sugar diet and a sedentary lifestyle) are strongly associated with PCOS as well and can lead to the issues described above.
Associated health complications
Infertility: As ovulation is affected in most individuals with PCOS, this may lead to infertility if not addressed. In fact, PCOS is one of the leading causes of infertility in women.
Pre-diabetes and type 2 diabetes: Women with PCOS have an increased risk of developing prediabetes and type-2 diabetes compared to women without PCOS. This risk is further increased by being overweight or obese, having insulin resistance or having an immediate family member with type-2 diabetes.
Metabolic syndrome and cardiovascular disease: Up to 80% of women with PCOS are overweight or obese. Both obesity and PCOS increase your risk for high blood sugar, high blood pressure, low HDL (“good”) cholesterol, and high LDL (“bad”) cholesterol. Together, these factors are called metabolic syndrome, and they increase the risk for heart disease, diabetes, and stroke.
Sleep apnea: Sleep apnea causes repeated pauses in breathing during the night that interrupt one’s sleep. It’s more common in women who are overweight, especially if they also have PCOS. Available evidence suggests that the high levels of androgens and obesity could be involved in the increased risk of sleep apnea in PCOS. There may even be a strong association between the severity of sleep apnea, glucose intolerance and insulin resistance.
Hair loss: The excess androgens in women with PCOS can be converted to a hormone called DHT (dihydrotestosterone), which promotes hair loss.
Acne: Testosterone, DHEA-S (androgens) and insulin stimulate the sebaceous glands in the skin to overproduce oil. This excess oil clogs the pores, which leads to bacterial growth, inflammation, and acne.
Managing PCOS
While the implications of PCOS seem dire, it is important to acknowledge the impact that lifestyle choices can have on this condition. For instance, insulin resistance is a common feature of PCOS and a causative factor for a number of its outcomes but can be dramatically reduced just through diet and exercise.
Lifestyle choices that can demonstrably help manage PCOS include:
A low carbohydrate diet: A comparison of studies on diets for PCOS have found that low-carbohydrate diets are effective for both weight loss and lowering insulin levels. A low glycemic index (low GI) diet, which includes carbohydrates from low GI fruits, vegetables, and whole grains, helps regulate the menstrual cycle better than a regular weight loss diet.
Consuming more fibre: Fibre can slow down the absorption of sugars from our food, and in doing so, regulates our insulin levels. Fibrous foods also increase satiety, i.e., make us feel full faster, which can help with weight loss.
Healthy fats: Omega-3 fatty acids (found in sources like fatty fish and avocados) can help lower inflammation in the ovaries (which can otherwise contribute to PCOS) and can even help with menstrual pain. Saturated fats, when consumed in moderation, can help regulate levels of leptin, the hormone that controls satiety. This can help regulate cravings, especially on a low GI diet.
Eating more vegetarian sources of protein: Experts suspect that excess consumption of commercially produced meat could increase testosterone levels (although these observations have been made in association with infertility rather than PCOS itself). Including some beans, nuts and seeds along with meat would offer not only additional plant-based protein, but some fibre too.
Healthy micronutrient status: Low levels of vitamin B12 have been associated with insulin resistance and other complications in PCOS patients. Scientists are not sure why, but supplementation with some B vitamins seems to help abate the symptoms of PCOS. Studies have suggested that low levels of vitamin D are linked to a larger fat mass, which is associated with resistance to leptin and insulin. Whether having low levels of vitamin D is the cause or effect of a higher fat mass is unclear, but it is important to ensure we have enough of it. Although sunlight is the best source, small amounts of vitamin D (especially D3) are also found in cheese, egg yolks, fatty fish, liver and mushrooms.
Exercise: According to few studies, performing moderate-intensity exercises for 30 minutes at least 3 days a week can help women with PCOS lose weight. This also improves ovulation and insulin levels.
While researchers continue to dig deeper into the factors that lead to PCOS in the first place, we do know that a healthy diet and exercise can help manage it. Let’s take charge of these two aspects of our life- for more reasons than one.
References:
Sirmans, S. M., & Pate, K. A. (2014). Clinical epidemiology, 6 (1).
https://www.britannica.com/science/ovary-animal-and-human
Holesh, J., & Lord, M. (2017). Physiology, Ovulation.
Yildiz, B. O., Carmina, E., & Azziz, R. (2006).
Begawy, A. F., El-Mazny, A. N., Abou-Salem, N. A., & El-Taweel, N. E. (2010). Middle East Fertility Society Journal, 15(4).
González, F. (2012). Steroids, 77(4)
Palomba, S., Santagni, S., Falbo, A., & La Sala, G. B. (2015). International journal of women’s health, 7.
Rubin, K. H., Glintborg, D., Nybo, M., Abrahamsen, B., & Andersen, M. (2017). The Journal of Clinical Endocrinology & Metabolism, 102(10).
Duleba, A. J., & Dokras, A. (2012).Fertility and sterility, 97(1).
Sam, S. (2007). Obesity Management, 3(2).
Tasali, E., Van Cauter, E., & Ehrmann, D. A. (2008). Sleep medicine clinics, 3(1).
Dumesic, D. A., & Lobo, R. A. (2013). Steroids, 78(8).
Herskovitz, I., & Tosti, A. (2013). International Journal of Endocrinology and Metabolism, 11(4).
Acne overview: National Library of Medicine. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0072395/
Dunaif, A., Graf, M., Mandeli, J., Laumas, V., & Dobrjansky, A. (1987). The Journal of Clinical Endocrinology & Metabolism, 65(3).
Zangeneh, F. Z., Jafarabadi, M., Naghizadeh, M. M., Abedinia, N., & Haghollahi, F. (2012). Journal of reproduction & infertility, 13(2).
Moran, L. J., Ko, H., Misso, M., Marsh, K., Noakes, M., Talbot, M., … & Teede, H. J. (2013). Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. Journal of the Academy of Nutrition and Dietetics, 113(4), 520-545.
Venn BJ, Mann JI. Eur J Clin Nutr 2004, 58(11): 1443-1461.
Chakrabarti J. Annals of Medical and Health Sciences Research 2013, 3(2): 191-196.
Pourghassem Gargari B, et al. International Journal of Fertility & Sterility 2015, 9(3): 313-321.
Tokuyama S, Nakamoto K. Biol Pharm Bull 2011, 34(8): 1174-1178.
Chavarro JE, et al. Am J Obstet Gynecol 2008, 198(2): 210.e211-217.
Chavarro JE, et al. Obstet Gynecol 2007, 110(5): 1050-1058.
Chehade JM, et al. Diabetes Spectrum 2009, 22(4): 214-218.
Harrison, C. L., Lombard, C. B., Moran, L. J., & Teede, H. J. (2010). Exercise therapy in polycystic ovary syndrome: a systematic review. Human Reproduction Update, 17(2), 171-183.
Raja-Khan N, et al. American Journal of Physiology – Endocrinology and Metabolism 2011, 301(1): E1-E10